Who are OrthoSolutions
We are a specialist orthopedic foot and ankle company that works in partnership with surgeons to design and develop implants and instrumentation for patients all over the world.
As one of the longest established foot and ankle specialists, we bring more than 20 years’ experience of working with leading surgeons to deliver evidence-based advances that enable better patient results. We back this up with world class training, education and support that helps our surgeons to excel.

System 26 Cannulated Screw System
One screw system. All your fixation needs. Comprehensive extremity screw system that facilitates bone fixation from forefoot to the ankle.
Learn More
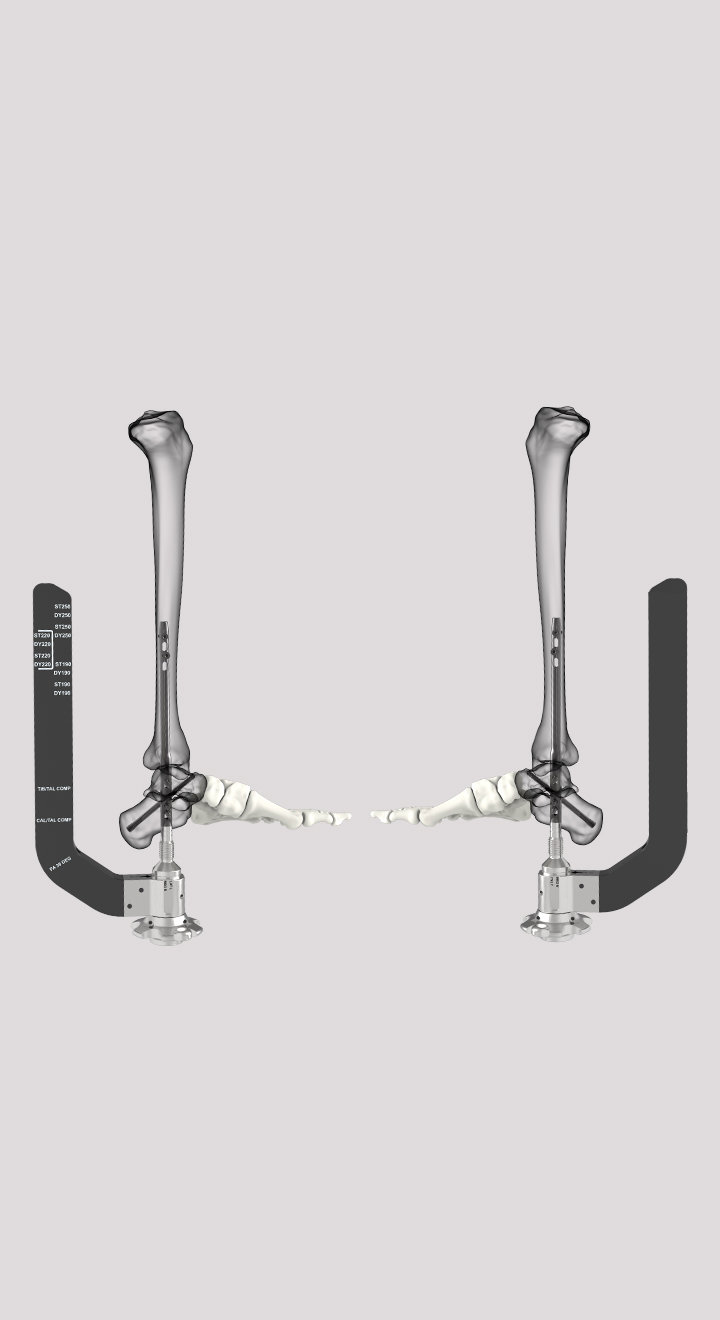
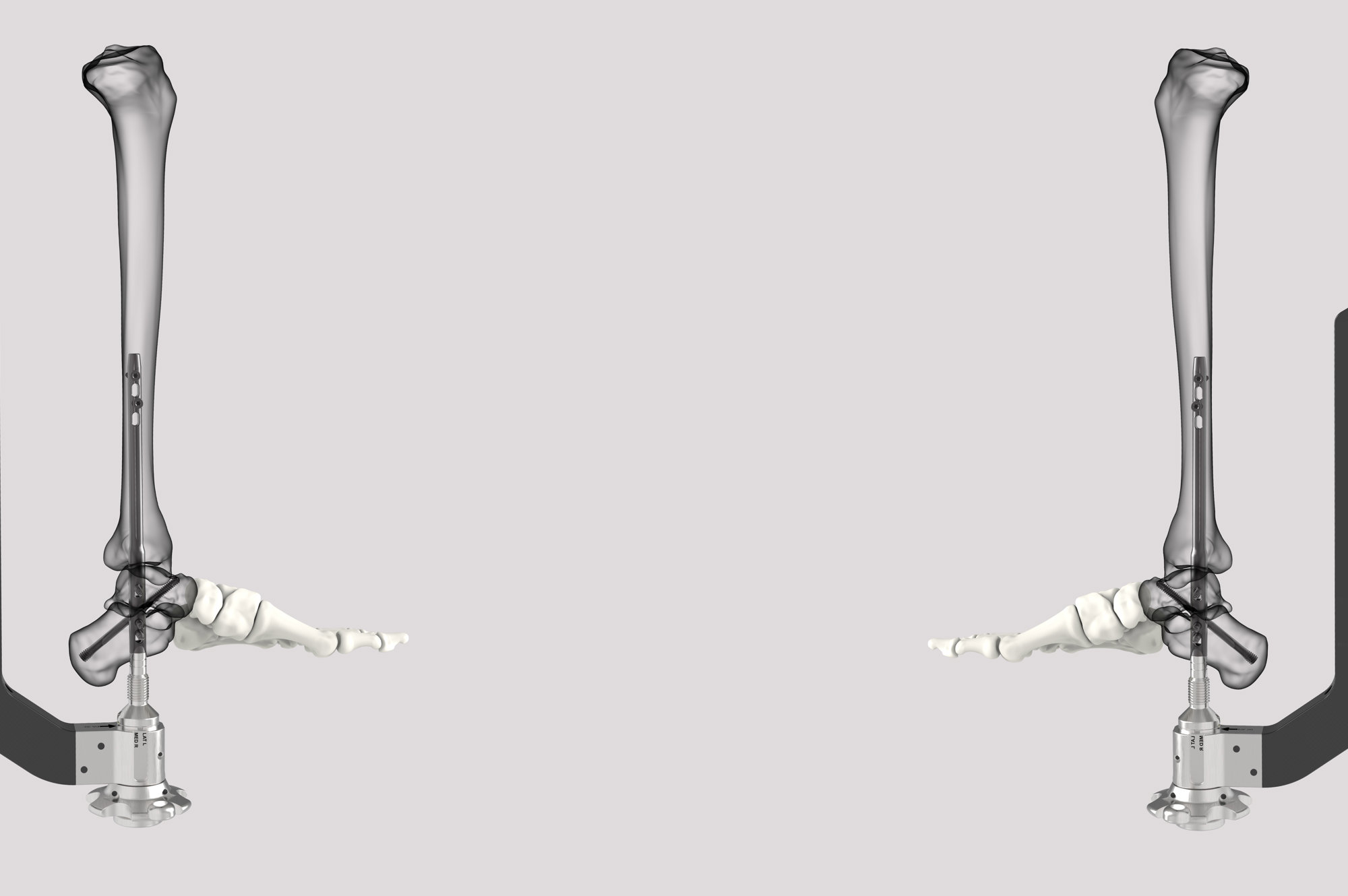
SUCCESSION® TTC Nail System
The SUCCESSION® TTC Nail System provides the surgeon with greater control, allowing for customized treatment based on each patient’s specific needs
Learn More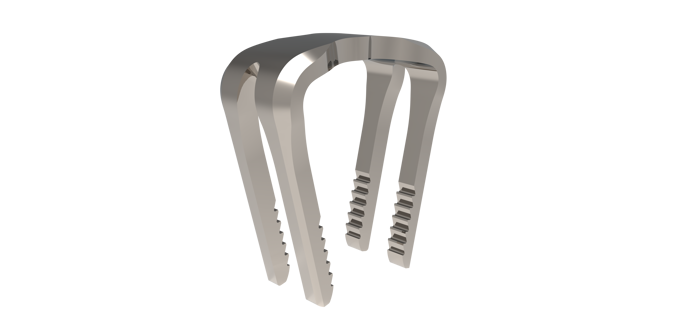
COGNiTiON™ Staple System
COGNiTiON™ STAPLE SYSTEM The staple that redefines strength and stability.
Learn More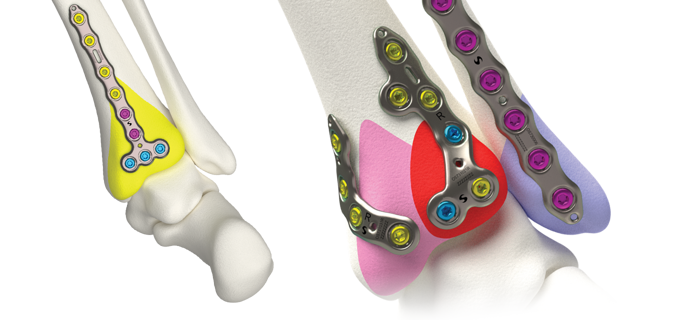
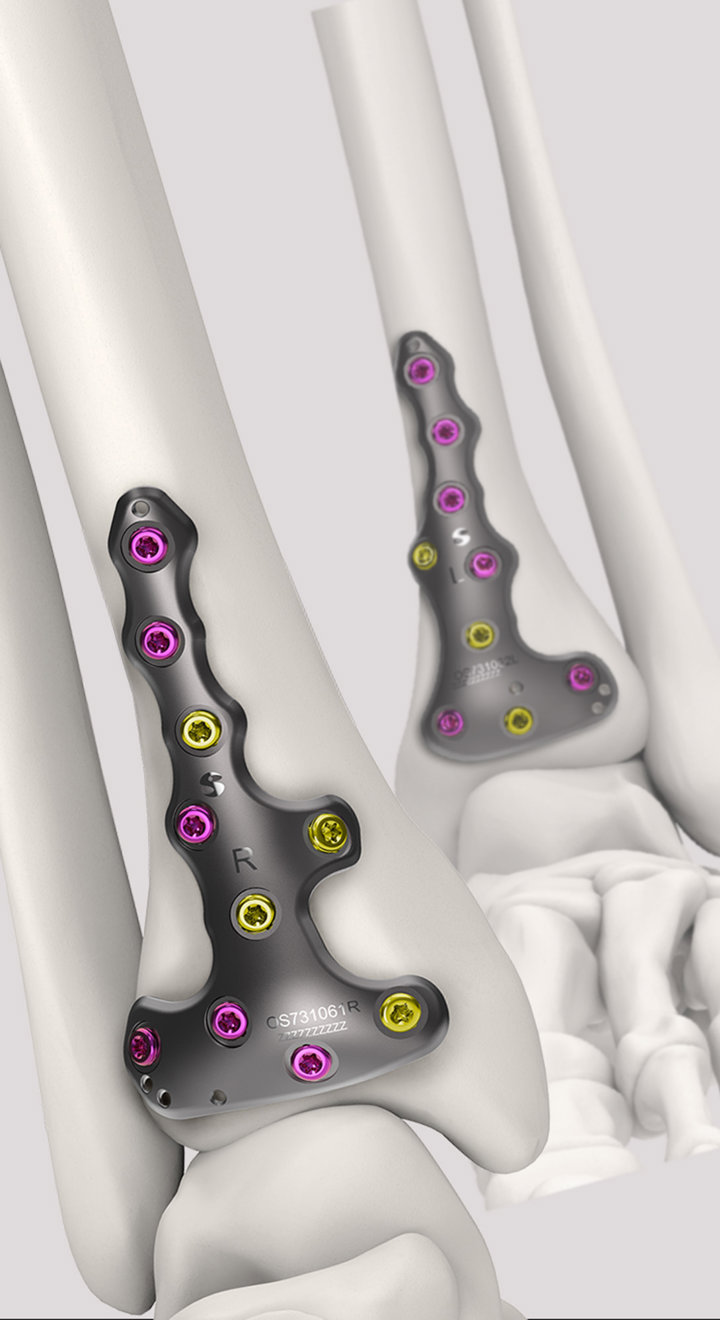
Volition® Ankle Fracture Plating
Evidence based ankle fracture plating solution. Anatomically designed to optimize the treatment of all types of ankle fractures.
Learn More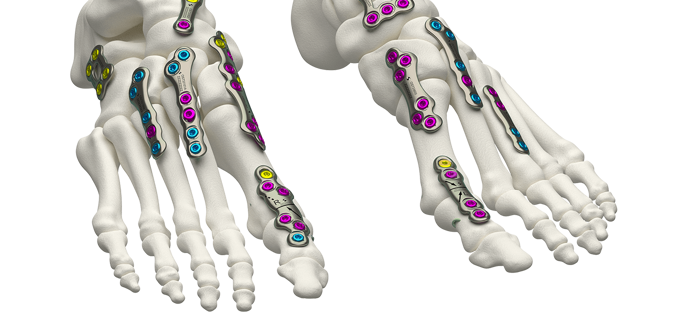
Volition® MTPJ and Universal Plating System
VOLITION® MTPJ and Universal plating system focuses on providing a solution for an array of conditions within the forefoot and midfoot.
Learn MoreWhat makes us different
Our goal is to deliver better patient outcomes. It’s a continuous process that never ends. Our refreshingly friendly and nurturing approach encourages and inspires our community of surgeons, healthcare professionals and employees to continually seek better ways of improving patients’ lives.